Signatera™ is a personalized circulating tumor DNA (ctDNA) test primarily used to monitor cancer in patients who have already been diagnosed.
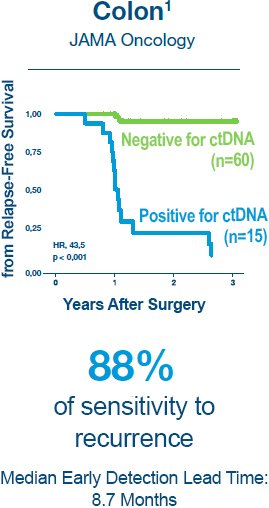
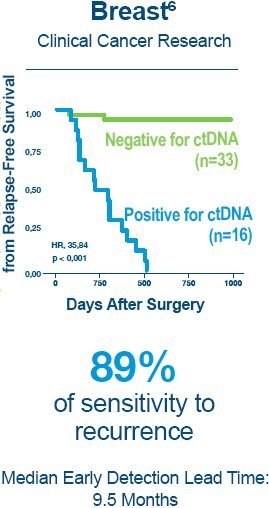
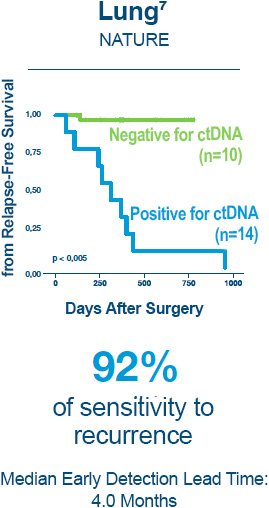
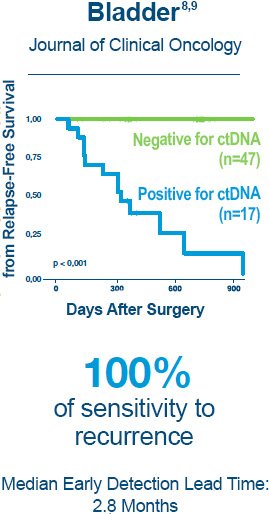
1. Reinert T, Henriksen TV, Christensen E, et al. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stages I to III Colorectal Cancer. JAMA Oncol. 2019.
2. Sinicrope FA, Foster NR, Thibodeau SN, et al. DNA Mismatch Repair Status and Colon Cancer Recurrence and Survival in Clinical Trials of 5-Fluorouracil-Based Adjuvant Therapy. J Natl Cancer Inst. 2011;103(11):863-875.
3. Aoyama, Oba K, Honda M, et al. Impact of postoperative complications on the collorectal cancer survival and recurrence: analyses of pooled individual patients’ data from three large phase III randomized trials. Cancer Med. 2017;6(7):1573-1580.
4. Yothers G, O’Connell MJ, Lopatin M, et al. Validation of the 12-gene colon cancer recurrence score in NSABP C-07 as a predictor of recurrence in patients with stage II and III colon cancer treated with fluorouracil and leucovorin (FU/LV) and FU/LV plus oxaliplatin, J Clin Oncol. 2013;31(36):4512-4519.
5. Kotani D. et al., Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer, Nature Medicine v29 Issue 1 Jan 2023
6. Coombes RC, Page K, Salari R, et al. Personalized Detection of Circulating Tumor DNA Antedates Breast Cancer Metastatic Recurrence. Clin Cancer Res. 2019;25(14):4255-4263.
7. Abbosh C, Birkbak NJ, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545(7655):446-451.
8. Christensen E, Birkenkamp-Demtroder K, Sethi H, et al. Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients With Urothelial Bladder Carcinoma. J Clin Oncol. 2019;37(18):1547-1557.
9. Data on file
10. Japanese Society of Medical Oncology Guidelines, October 2022.
Consider this test in the following cases:
Use Signatera™ in the neoadjuvant context to identify and support a “watch and wait” or non-surgical approach in specific patients.
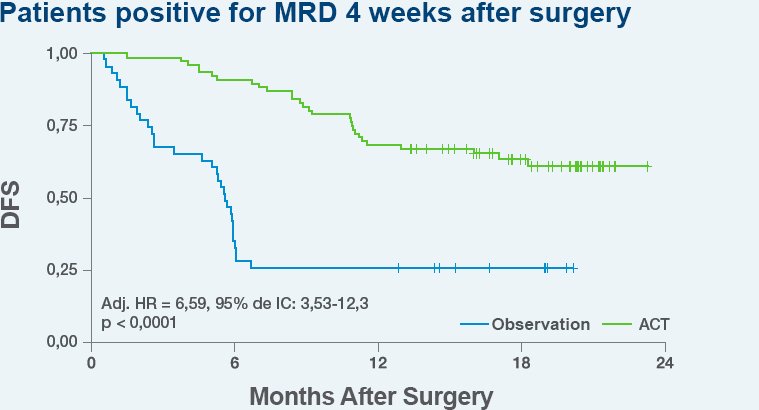
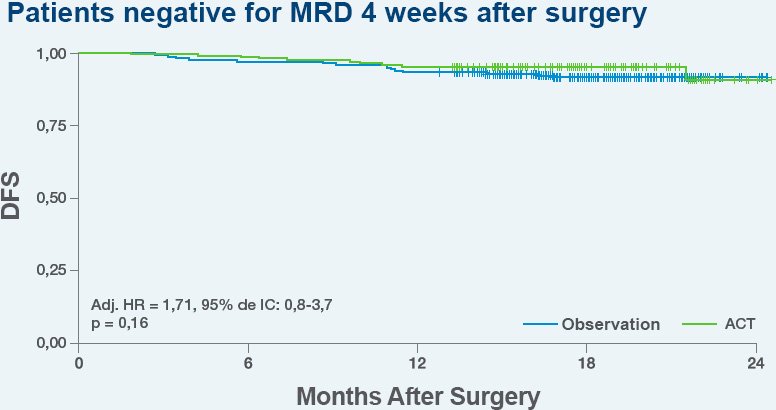
Use Signatera™ after surgery to assess the need for adjuvant chemotherapy and potentially avoid unnecessary treatment.
Use Signatera™ alongside CEA tests and imaging to detect recurrence earlier.
Signatera™ enables real-time monitoring of ctDNA dynamics for early and sensitive evaluation of treatment response.
Tumor Exome
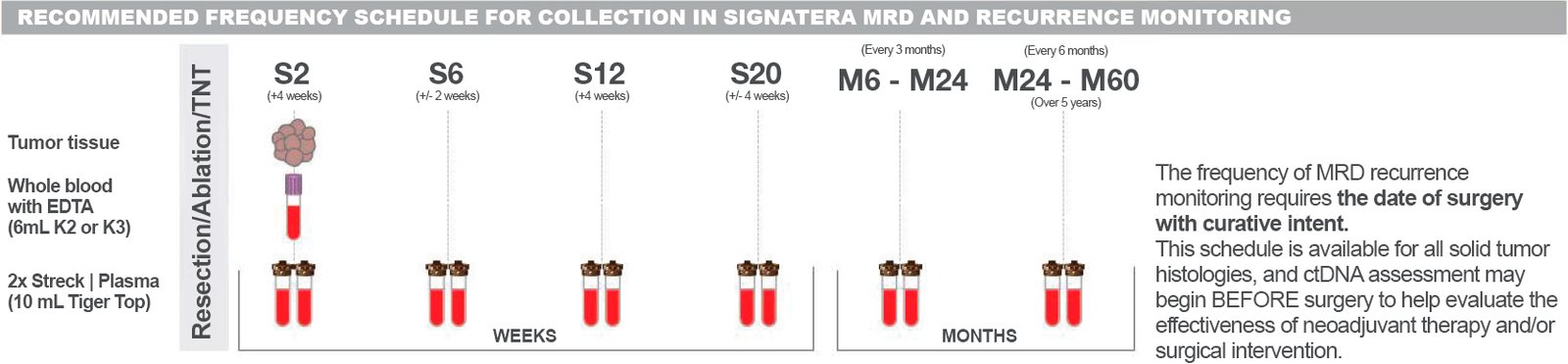
The methodology used for this test is as follows:
Blood: No fasting required.
Biopsy: In Paraffin Block or Slide.
Tumor Exome – Up to 40 calendar days
Monitoring – Up to 12 calendar days
Medical request.
| Type | Description |
| Technique | ctDNA |
| Accuracy | By tracking only tumor-specific variants, sensitivity is maximized with a low limit of detection (LOD) of 0.01% variant allele frequency (VAF). Additionally, Signatera™ filters clonal hematopoiesis of indeterminate potential (CHIP) mutations to greatly reduce false positives. |

Main Office Rua Bento Gonçalves, 59, Room 802 — Centro, Marau, RS — 99150-000
Porto Alegre Office Rua Gomes Jardim, 301 , Room 918/909 — Santana, Porto Alegre, RS — 90620-130