
Oncotype DX is a genomic test used to assess the risk of recurrence in early-stage breast cancer and assist in deciding on the use of adjuvant chemotherapy. In addition to its widespread use in breast cancer, there are versions of the test for prostate and colon cancer.

Consider this test in the following cases:
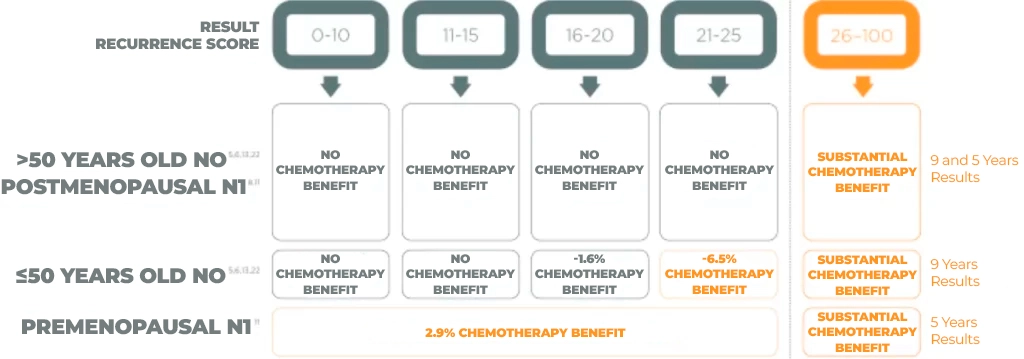
21 genes, including 16 tumor-related genes and 5 control genes, to generate a Recurrence Score.
The methodology used for this test is as follows:
Biopsy: In Paraffin Block or Slide
Up to 30 calendar days
Medical request.

| Type | Description |
| Technique | RT-PCR |
| Accuracy | 85 – 90% |

Main Office Rua Bento Gonçalves, 59, Room 802 — Centro, Marau, RS — 99150-000
Porto Alegre Office Rua Gomes Jardim, 301 , Room 918/909 — Santana, Porto Alegre, RS — 90620-130